electron configuration for lithium|electron configuration chart : Baguio Mar 23, 2023 Having a wide variety of games in store, Dafabet Casino offers only the best online slots and live games to our avid bettors. We aim to provide a seamless online gaming experience to our valued players and with that, we continuously maintain a high level data security on top of the assured fair gaming environment.
PH0 · write the electron configuration for lithium
PH1 · which is the electron configuration for boron
PH2 · lithium ground state electron configuration
PH3 · electron configuration list
PH4 · electron configuration guide
PH5 · electron configuration explained
PH6 · electron configuration chart
PH7 · electron configuration calculator
PH8 · Iba pa
Rendam Sex Full XXX Videos, Porn HD, Free XXX Clips, HQ Sex Tube, Best Xvideos
electron configuration for lithium*******Mar 23, 2023
Click on above elements (in Periodic table) to see their information or Visit .Lithium is the third element with a total of 3 electrons. In writing the electron configuration for lithium the first two electrons will go in the 1s orbital. Since 1s can .
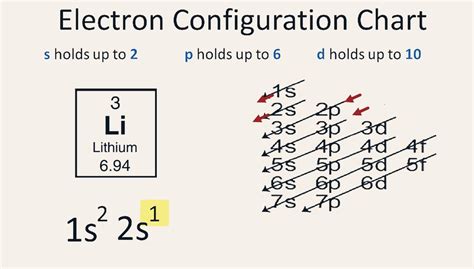
A step-by-step description of how to write the electron configuration for Lithium (Li). In order to write the Li electron configuration we first need to know the number of electrons for.electron configuration for lithium A step-by-step description of how to write the electron configuration for Lithium (Li). In order to write the Li electron configuration we first need to know the number of electrons for.
Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. . Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s .The electron configuration of an element is the arrangement of its electrons in its atomic orbitals. By knowing the electron configuration of an element, we can predict and .2.6: Electron Configurations. Page ID. OpenStax. Learning Objectives. Give the electron configuration for an atom using Bohr’s model, box orbital diagrams, and quantum mechanical notation. Bohr's Model. Here . The electron configuration of Lithium (Li) is 1s2 2s1. Li is a silvery white metal. Its atomic weight is 6.941 u. Li readily oxidizes to lithium oxide on exposure to .
electron configuration chartGoogle Classroom. What are electron configurations? The cells in our bodies are masters of quantum physics---they’ve figured out the complicated dance of atoms and electrons, . Because lithium’s final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s 2 2s 1. The shell diagram for a lithium atom (Figure \(\PageIndex{1}\)). The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1 s , while the outermost shell ( 2 s ) has 1 electron.
electron configuration for lithium electron configuration chartTwo of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s orbital. Therefore, we write the electron configuration of a lithium atom as 1s 2 2s 1 (spoken as “one-ess-two two-ess-one”). The shell diagram for a lithium atom (Figure \(\PageIndex .Sarah Faizi (University of California Davis) 2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is .For hydrogen, therefore, the single electron is placed in the 1s orbital, and the electron configuration (also known a spdf notation) . Using this notation to compare the electron configurations of sodium and lithium, we have: Sodium: 1s 2 2s 2 2p 6 3s 1 = [Ne]3s 1: Lithium: 1s 2 2s 1 = [He]2s 1:Its electron configuration is. He: 1s2 He: 1 s 2. The three electrons for Li are arranged in the 1s subshell (two electrons) and the 2s subshell (one electron). The electron configuration of Li is. Li: 1s22s1 Li: 1 s 2 2 s 1. Be has four electrons, two in the 1s subshell and two in the 2s subshell.
The electron configuration and orbital diagram of helium are: The n = 1 shell is completely filled in a helium atom. The next atom is the alkali metal lithium with an atomic number of 3. The first two electrons in lithium fill the 1s orbital and have the same sets ofThe electron configuration of lithium is [He] 2s1. . All elements are identified by their atomic number Z, which is the same as the number of protons and electrons in their nucleus. In the case of lithium, it corresponds to atomic number 3. In this case, the lithium atom has 3 electrons, two of them fill the 1s orbital, while the third one goes . Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s orbital. Therefore, we write the electron configuration of a lithium atom as 1s 2 2s 1 (spoken as “one-ess-two two-ess-one”). The shell diagram for a lithium atom (Figure \(\PageIndex .
Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. The electron configuration for the first 10 elements. H .Two of the lithium electrons can fit into the 1s subshell, but the third electron must go into the second shell and the lower energy orbital, which is the 2s orbital. Therefore, we write the electron configuration of a lithium atom as 1s 2 2s 1 (spoken as “one-ess-two two-ess-one”). The shell diagram for a lithium atom (Figure \(\PageIndex .
Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 2.6.6 2.6. 6. The valence shells of the inner transition elements consist of the ( n – 2) . This means that a neutral lithium atom will have a total of 3 electrons surrounding its nucleus. Its electron configuration will be. Li: 1s22s1. Now, the lithium cation, Li+, is formed when lithium loses the .
Answer: The electron configurations of the elements are presented in Figure 2.2.3, which lists the orbitals in the order in which they are filled. In several cases, the ground state electron configurations are different from those predicted by Figure 2.2.1. Some of these anomalies occur as the 3 d orbitals are filled. Lithium is an alkali metal with the atomic number = 3 and an atomic mass of 6.941 g/mol. This means that lithium has 3 protons, 3 electrons and 4 neutrons (6.941 - 3 = ~4). Being an alkali metal, lithium is a soft, flammable, and highly reactive metal that tends to form hydroxides. It also has a pretty low density and under standard conditions . The electronic ground state for lithium is 1 s2 2 s, and not 1 s2 2 p. The traditional argument for why this is so is based on a screening argument that claims that the 2 p electron is better shielded by the 1 s electrons, and therefore higher in energy than the configuration that includes the 2 s electron. We show that this argument is flawed .

Because lithium’s final electron goes into the 2s subshell, we write the electron configuration of a lithium atom as 1s 2 2s 1. The shell diagram for a lithium atom is shown below. The shell closest to the nucleus (first shell) has 2 dots representing the 2 electrons in 1 s , while the outermost shell ( 2 s ) has 1 electron. Electron configurations of ions. To find the electron configuration for an ion, first identify the configuration for the neutral atom. Then, add or remove electrons depending on the ion's charge. For example, to find the configuration for the lithium ion (Li⁺), start with neutral lithium (1s²2s¹). Then, since the lithium ion has one less .
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).
Amsteradam RAI, The Netherlands. Start Date. Tuesday, February 5, 2019 - 08:00
electron configuration for lithium|electron configuration chart